Company News
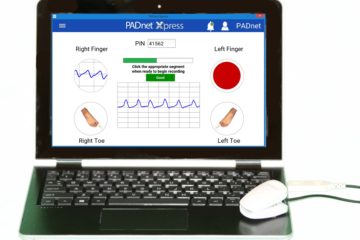
New published whitepaper establishes clinical accuracy of PADnet Xpress
Lower Extremity Review magazine published a whitepaper online highlighting a clinical study comparing the diagnostic results of PADnet Xpress with other established, gold-standard technologies used to perform physiologic studies to non-invasively detect peripheral artery disease (PAD). This whitepaper was authored by Sue Duval, PhD and Associate Professor of the University Read more…